
#Stem cell treatment for diabetes type 1 cost Activator#
Proinflammatory cytokines further induce signal transducer and activator of transcription 1, nuclear factor κB, and interferon regulatory factor 3 in β-cells, contributing to the maintenance and amplification of the immune processes. IFN-γ may also activate macrophages to release proinflammatory cytokines and reactive oxygen species (ROS). IL-2 and proinflammatory cytokines released by activated CD4 T cells (e.g., interferon-γ, tumor necrosis factor, and IL-1β) maximize the activation of cytotoxic CD8 T cells, the final effectors of β-cell death via apoptosis.
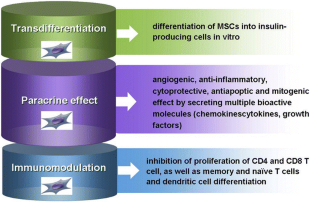
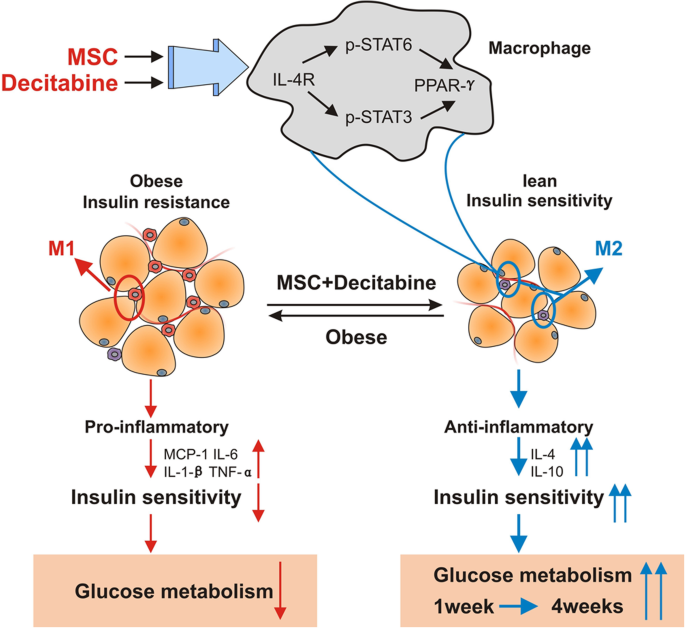
Studies indicate that interleukin (IL)-12 secreted by macrophages may activate Th1-type CD4 T cells. Antigen-presenting cells such as macrophages and dendritic cells (DCs) are the first to infiltrate islets followed by CD4 and CD8 T lymphocytes, natural killer (NK) cells, and B lymphocytes. Autoantibodies against islet antigens are a hallmark of disease development. Immunopathogenesis of T1DĪ combination of environmental risk factors, genetic predisposition, and autoimmune-mediated processes contribute to T1D etiology. This review will focus on the role of stem cells in diabetes cell therapy, with emphasis on bone marrow-derived hematopoietic stem cells (BM-HSCs) and multipotent mesenchymal stromal cells (MSCs). Therefore, understanding the immunopathogenesis of T1D is crucial for designing effective β-cell replacement and immunomodulatory strategies. Failure of interventional therapies in preventing autoimmune β-cell destruction can be attributed to a number of issues such as the transient nature of immune protection that often results in the recurrence of autoimmunity upon drug withdrawal and the failure to induce a tolerant state. Although significant advancement in our understanding of T1D immunopathogenesis has occurred since the efficacy of cyclosporine in reducing insulin requirement was reported more than 25 years ago, immunomodulatory therapies since then have not met with expected clinical success. Since 70%–90% of β-cells have been destroyed at the time of diagnosis, the impact of strategies that aim at preserving β-cell mass is limited. The ideal goal of clinical intervention would be to prevent or arrest the onset and progression of autoimmunity, reverse β-cell destruction, and restore glycometabolic control and immune homeostasis. Type 1 diabetes mellitus (T1D) is a chronic, multifactorial autoimmune disease that involves the progressive destruction of pancreatic β-cells, ultimately resulting in the loss of insulin production and secretion. To conclude, the application of stem cell therapy in the cure for T1D appears extremely promising. The benefits of combinatorial approaches designed to ensure the successful clinical translation of stem cell therapeutic strategies, such as approaches combining effective stem cell strategies with islet transplantation, immunomodulatory drug regimens, and/or novel bioengineering techniques, are also discussed.

Herein, we discuss the therapeutic potential of stem cells derived from a variety of sources for the cure of T1D, for example, embryonic stem cells, induced pluripotent stem cells, bone marrow-derived hematopoietic stem cells, and multipotent mesenchymal stromal cells derived from bone marrow, umbilical cord blood, and adipose tissue. While the regenerative potential of stem cells can be harnessed to make available a self-replenishing supply of glucose-responsive insulin-producing cells, their immunomodulatory properties may potentially be used to prevent, arrest, or reverse autoimmunity, ameliorate innate/alloimmune graft rejection, and prevent recurrence of the disease. Stem cell therapy offers a solution to the cited challenges of islet transplantation. Despite promising outcomes observed with islet transplantation and advancements in immunomodulatory therapies, the need for an effective cell replacement strategy for curing T1D still persists. The goal of clinical intervention is to prevent or arrest the onset and progression of autoimmunity, reverse β-cell destruction, and restore glycometabolic and immune homeostasis.


 0 kommentar(er)
0 kommentar(er)
